Automatic Respiration and Ondine's Curse
The act of breathing is often automatic and unforced. However, the respiratory physiology that underpins automatic, unconscious breathing is complex, with scientific consensus yet to be reached regarding many of its aspects.
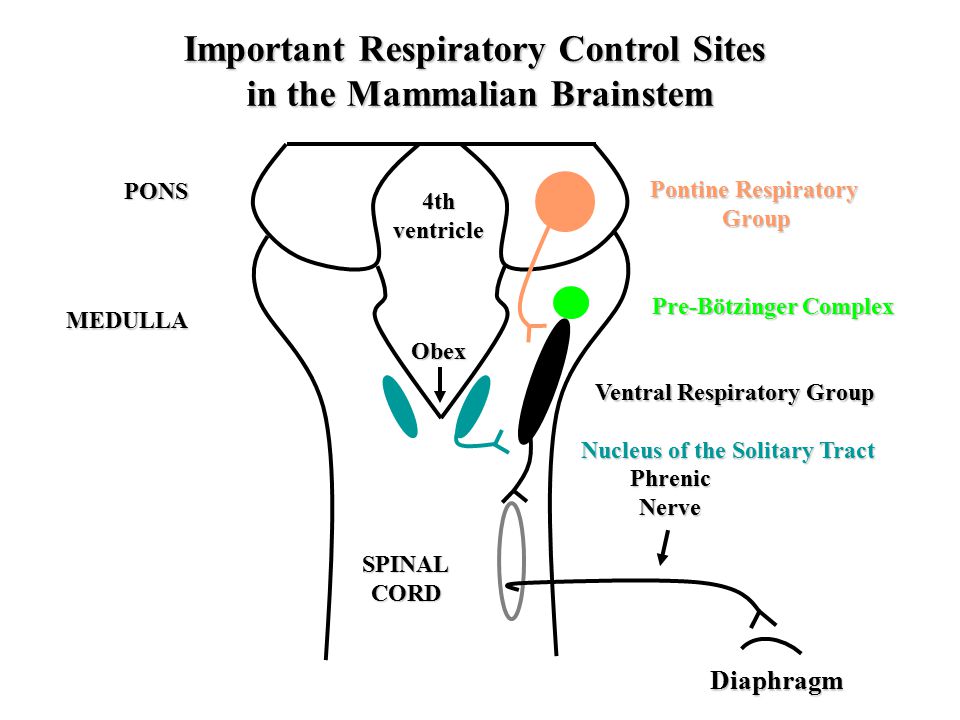
Withing these respiratory groups clusters of respiratory neurons are found, so-called due to firing at a distinctive point of the respiratory cycle. Respiratory are key in modulating the respiratory cycle, while some are essential in generating the respiratory rhythm.
In 1873 Legallois identified the kernel of breathing control to be at the level of brainstem, breathing continued even with the removal of the cerebrum.
In the 1980s, the repiratory area was narrowed to the Pons and Medulla of the brainstem,
this was discovered following in vivo experimentation in cats, as surgical removal of the brain below this level was followed by a cessation of the automatic respiratory rhythm.
This subsequently led to the discovery of the pontine, ventral and dorsal respiratory groups (the PRG, VRG and respectively).
Within the PRG, the Kolliker-Fuse and the parabrachial nucleui are involved in both the inspiratory and expiratory phase respiration with output directly to the phrenic motor nuclei or indirectly through the VRG or DRG of the medulla.
Within the VRG, both insipratory and expiratory bulbospinal nuclei are found (pre-motor neurones modulating respiratory rhythm), projecting predominately to the phrenic motor nuclei, external and internal intercostal motoneurones respectively (motor neurones which directly innervate skeletal muscles essential for respiration e.g. diaphragm)
However, uniquely VRG also contains propriobulbar neurones which project locally, one cluster of such neurones have been identified as essential in generating the respiratory rhythm. This cliucter of interneurones have been named, the Pre-Botzinger Complex (PreBotC), studies by Smith et al., 1991, showed that addition DAMGO, the μ-opioid receptor agonist to this area resulted in decreased firing in vitro. Conversely addition of the neuropeptide Substance P, binding at the Neurokinin receptor 1 (NKR1) increased the rate of firing. NKR1 in particular has led to the isolation of anatomic structure, as PreBotC neurones are unique as the only respiratory group neurones in the brainstem to express the NKR1 receptor (Gray, 1999).
Ondine's curse is carachterised by a lack of automatic breathing, with volition control still being in tact. Aquired forms of this Central Hypoventilation Syndrome can follow trauma to the brainstem region. Congenital Central Hypoventilation Syndrome (CCHS) is often if undetected, as children severely hypoventilate during sleep and some can even present with prolonged aponea. There is also severe reduction of chemosenstivty, with bunted responce to hypoxia and hypercapnia.. Genetic mutation of the transcriptional factor Phox2b has been identified as the root cause of CCHS.
Current treatment includes implanted electrical stimulation of the diaphragm as well as using ventilator during sleep.
Animal models have been difficult to produce as deletion of the Phox2b gene has been too severe and not viable, with a complete lack of sympathetic, parasympathetic and enteric ganglia.
In order to mimic human condition, a model confining symptom to the respiratory system is needed with Transgenic mice generated with the most frequent of the CCHS-causing mutations,
a +7 Alanine expansion of the 20-residue polyAla tract by a knock-in approach.
The mice specifically lack a population of glutamatergic Phox2b-expressing neurones in
the RTN/pFRG.
All other structures that depend on Phox2b for normal development, e.g. carotid body
are normal. Transgenic mutant pups have severely disrupted breathing with seveal aponea, they also do not respond to hypercapnia and there is no increase in ventilation with an increase in PCO2 .
In Phox2b trasngenic models, fluorescent immunocytochemistry shows that PreBotC is intact and its function not affected unlike the RTN/pFRG. Thus, its can be concluded that it is indeed the chemosensing ability that is disrupted in CCHS rather than rhythm generation.
Though over the last few decades much has been elucidated in terms of rhythm generation and overall respiratory physiology. However, yet more needs to discovered in order to highlight potential therapies for debilitating conditions such as CCHS.
Within the VRG, both insipratory and expiratory bulbospinal nuclei are found (pre-motor neurones modulating respiratory rhythm), projecting predominately to the phrenic motor nuclei, external and internal intercostal motoneurones respectively (motor neurones which directly innervate skeletal muscles essential for respiration e.g. diaphragm)
However, uniquely VRG also contains propriobulbar neurones which project locally, one cluster of such neurones have been identified as essential in generating the respiratory rhythm. This cliucter of interneurones have been named, the Pre-Botzinger Complex (PreBotC), studies by Smith et al., 1991, showed that addition DAMGO, the μ-opioid receptor agonist to this area resulted in decreased firing in vitro. Conversely addition of the neuropeptide Substance P, binding at the Neurokinin receptor 1 (NKR1) increased the rate of firing. NKR1 in particular has led to the isolation of anatomic structure, as PreBotC neurones are unique as the only respiratory group neurones in the brainstem to express the NKR1 receptor (Gray, 1999).
Bilateral specific ablation of these neurones is achieved through conjugated carriage of Saporin (a ribosome inactivating protein) via Substance-P, which allows for saporin to enter PreBotC neurones and effectively kill neuronal cells. In vivo Stereotaxic surgery of animal models to achieve this has shown to result in severe apnoea during REM sleep (McKay et al., 2005), and overtime resulting in ataxic breathing. Though the respiratory rythmogenecity of the PreBotC has reached scientific consensus, the specific pathways for this is still contested and is of great interest in current scientific research.
Another group of potentially rhythm generating neruones have been identified in the rostral VRG is the retrotrapazoid nucleus/parafacial respiratory group (RTN/pFRG). Currently two opposing hypothesis exist: RTN/pFRG is an epiratory ryhtm generater and RTN/pFRG is essential for rhythm generation in development and early postnatal life - When the respiratory system matures, the preBötzinger complex becomes the dominant respiratory rhythm generating oscillator. However there is also evidence for RTN/pFRG and its role in chemosensitivity and NOT rhythm generation. There is currently however no evidence if there is integration between the PreBotC and the RTN/pFRG.
Ondine's Curse
Ondine's curse is carachterised by a lack of automatic breathing, with volition control still being in tact. Aquired forms of this Central Hypoventilation Syndrome can follow trauma to the brainstem region. Congenital Central Hypoventilation Syndrome (CCHS) is often if undetected, as children severely hypoventilate during sleep and some can even present with prolonged aponea. There is also severe reduction of chemosenstivty, with bunted responce to hypoxia and hypercapnia.. Genetic mutation of the transcriptional factor Phox2b has been identified as the root cause of CCHS.
Current treatment includes implanted electrical stimulation of the diaphragm as well as using ventilator during sleep.
Animal models have been difficult to produce as deletion of the Phox2b gene has been too severe and not viable, with a complete lack of sympathetic, parasympathetic and enteric ganglia.
In order to mimic human condition, a model confining symptom to the respiratory system is needed with Transgenic mice generated with the most frequent of the CCHS-causing mutations,
a +7 Alanine expansion of the 20-residue polyAla tract by a knock-in approach.
The mice specifically lack a population of glutamatergic Phox2b-expressing neurones in
the RTN/pFRG.
All other structures that depend on Phox2b for normal development, e.g. carotid body
are normal. Transgenic mutant pups have severely disrupted breathing with seveal aponea, they also do not respond to hypercapnia and there is no increase in ventilation with an increase in PCO2 .
In Phox2b trasngenic models, fluorescent immunocytochemistry shows that PreBotC is intact and its function not affected unlike the RTN/pFRG. Thus, its can be concluded that it is indeed the chemosensing ability that is disrupted in CCHS rather than rhythm generation.
Though over the last few decades much has been elucidated in terms of rhythm generation and overall respiratory physiology. However, yet more needs to discovered in order to highlight potential therapies for debilitating conditions such as CCHS.
Comments
Post a Comment